Watch The Recorded Webinar Below ||| Deal Open For Investment For A Limited Time
|||
Watch The Recorded Webinar Below ||| Deal Open For Investment For A Limited Time |||
A Smarter Electrosurgical Blade for a Safer Operating Room
A rare investment opportunity to help shape the operating room of tomorrow. From precision energy to real-time AI guidance — a complete surgical platform begins here.
Project Overview
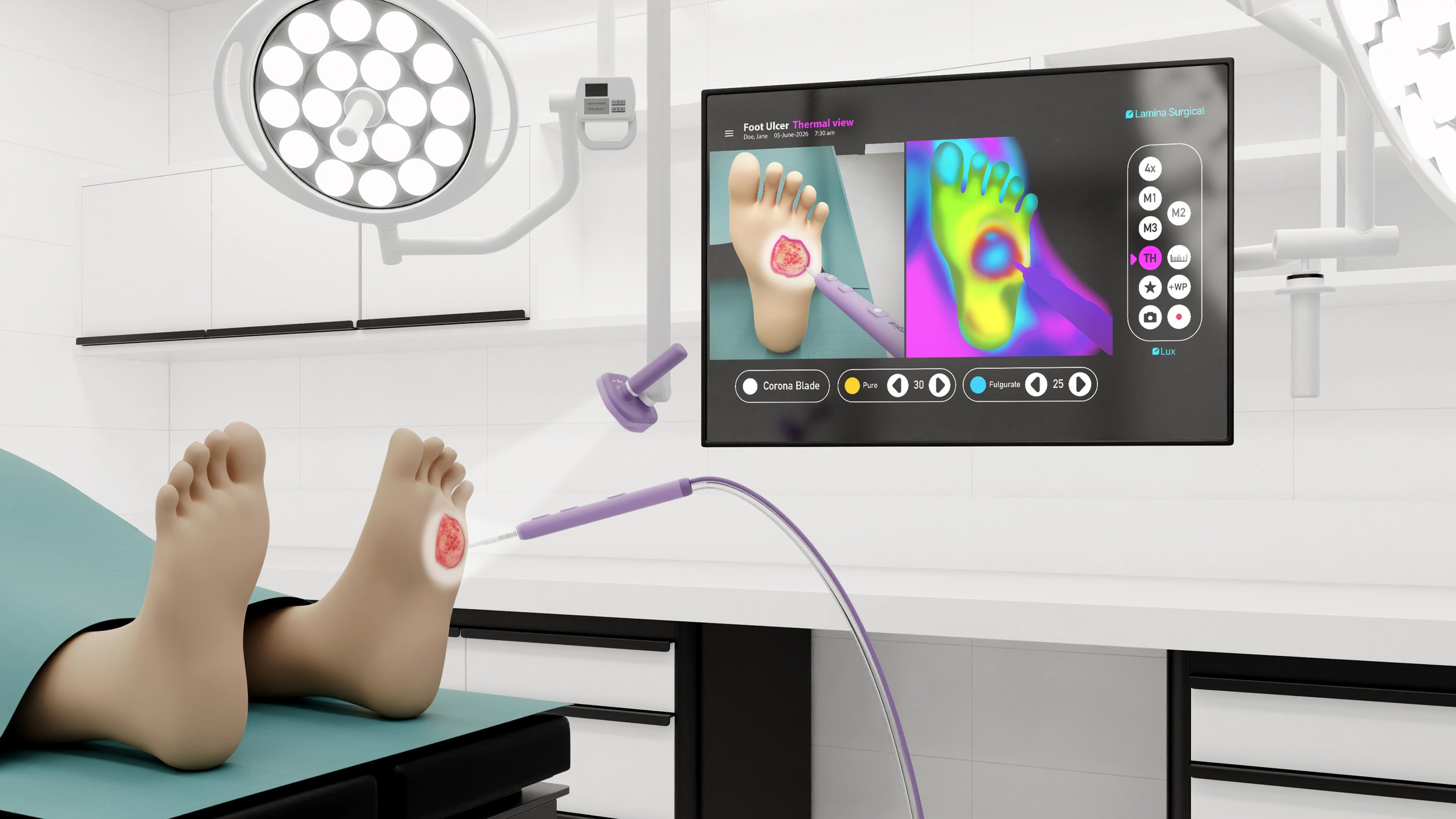
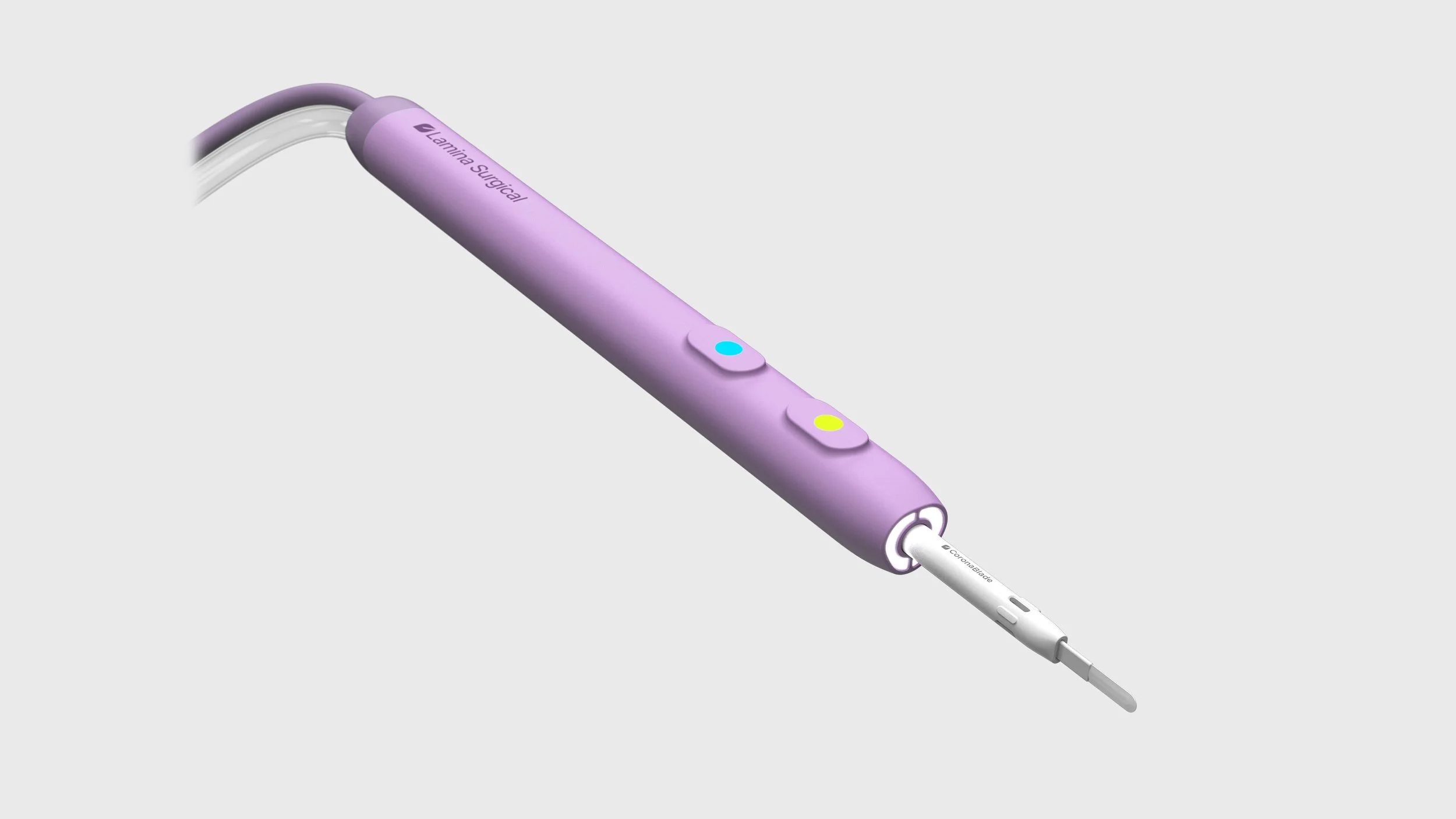
CoronaBlade is a next-generation electrosurgical instrument engineered to deliver cleaner cuts, superior coagulation, and dramatically reduced thermal spread—without requiring hospitals or ASCs to purchase capital equipment. CoronaBlade, built on Lamina Surgical technology designed by the engineers behind the PEAK PlasmaBlade™ and PhotonBlade, is positioned to become the new standard in open surgery instrumentation.
The Problem
Despite advances in minimally invasive surgery, open surgery remains a massive global volume category, and current electrosurgical tools still suffer from:
Excessive thermal spread → unintended tissue injury
Inconsistent coagulation performance → increased bleeding risk
Poor visualization and ergonomics → inefficient dissection
Expensive proprietary generators → adoption barriers in cost-sensitive settings
Surgeons need safer, more precise instruments that integrate seamlessly into existing workflows.
CoronaBlade improves on legacy electrosurgical design through:
The CoronaBlade Solution
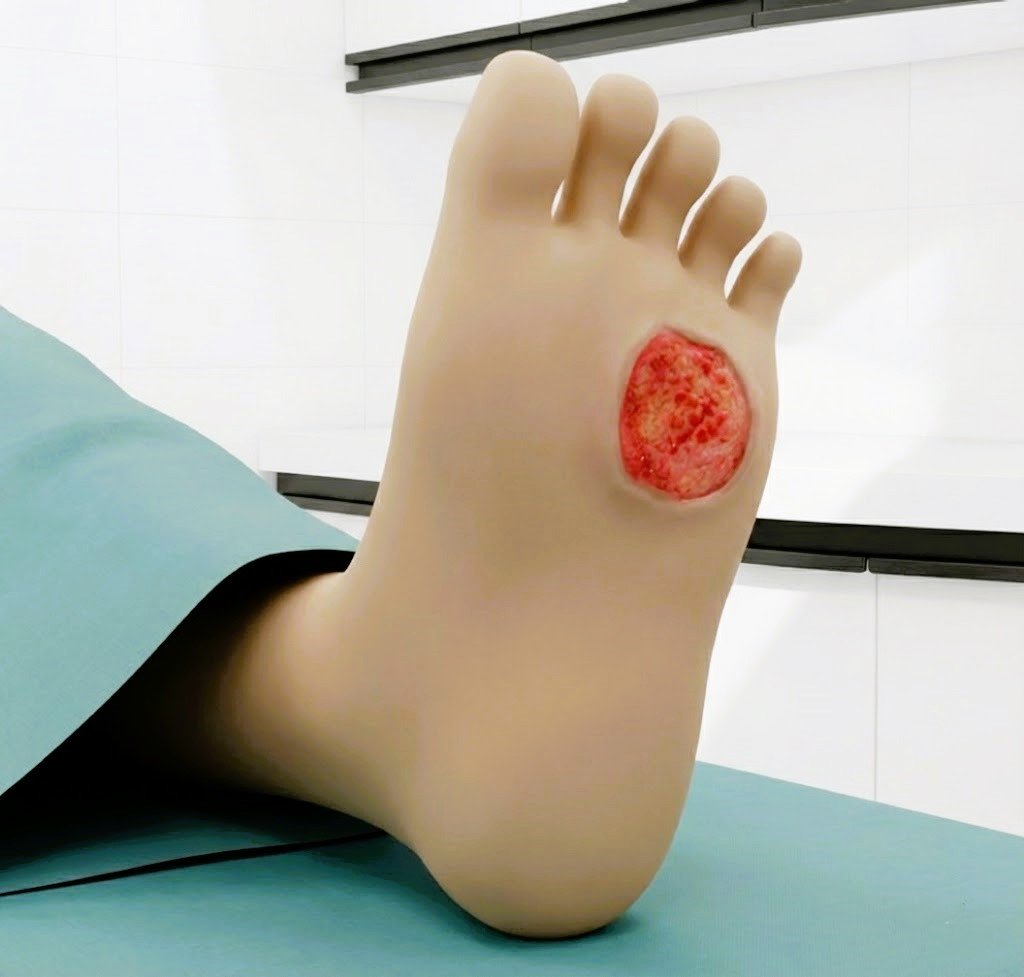
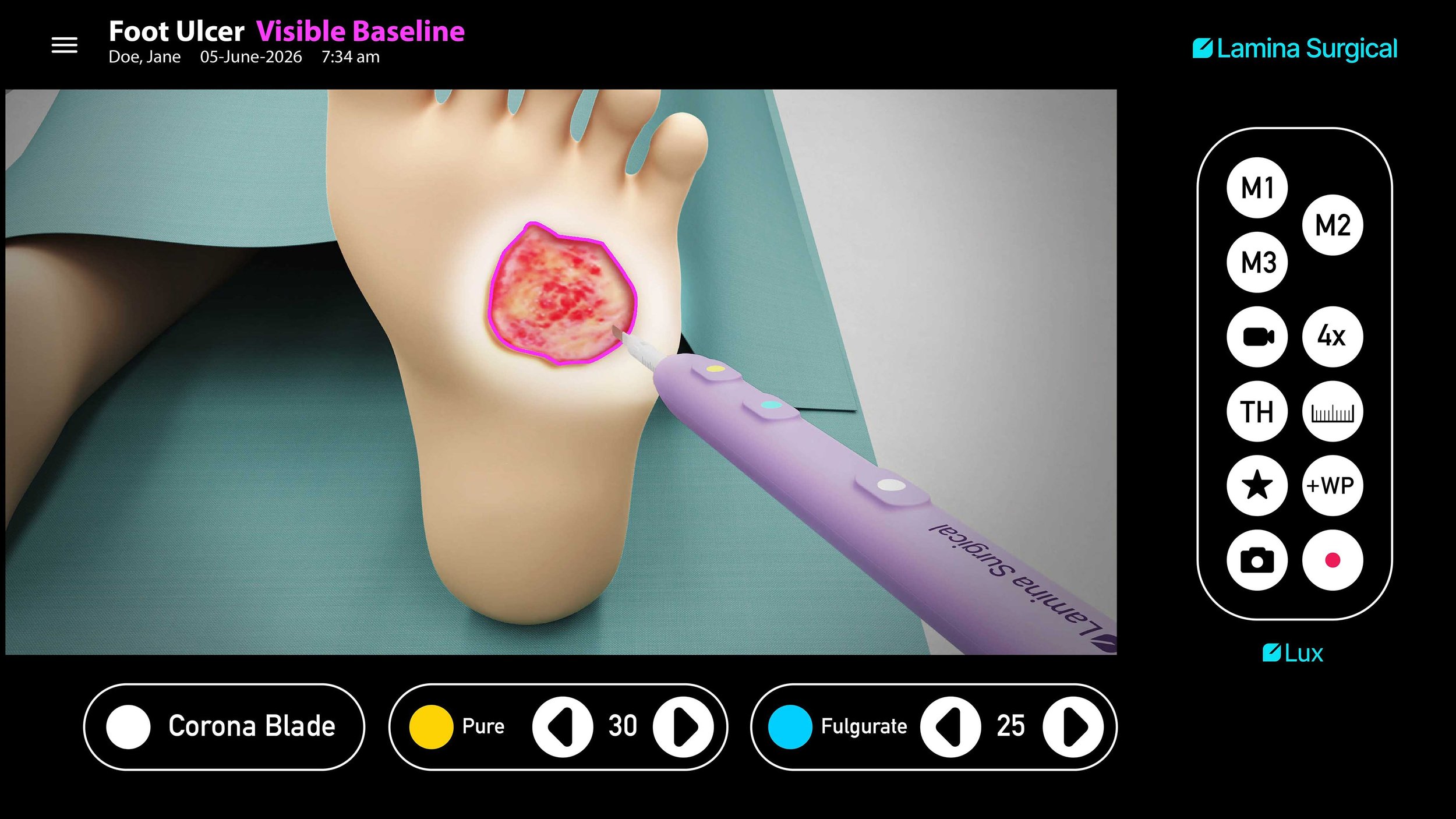
Glass-Coated Blade for Reduced Thermal Spread Minimizes collateral tissue damage and yields cleaner, more controlled dissection planes.
Advanced Energy Delivery & Superior Coagulation Improves hemostasis, reduces operative bleeding, and enhances surgeon confidence
Plug-and-Play Compatibility Works with existing Bovie RF generators—no need for hospitals to purchase $35K–$55K proprietary systems
Designed for Efficiency Improved ergonomics, reduced smoke, and optimized geometry support surgeon control and safety
CoronaBlade: The First Step In a New Surgical Ecosystem
CoronaBlade is the first step in a broader surgical platform that will integrate advanced imaging and AI. Upcoming phases aim to provide real-time tissue visualization, improved margin assessment, and eventually an AI-driven surgical copilot that offers guidance based on instrument–tissue interactions. Together, these innovations will transform CoronaBlade from a single device into a smarter, safer surgical ecosystem.

The CoronaBlade represents a rare early-stage opportunity backed by a world-class engineering team with a deep history of successful MedTech innovation, commercialization, and value creation.
Why We Love This Opportunity
-
CoronaBlade is led by veteran innovators behind some of the most impactful electrosurgical technologies in modern MedTech.
Paul Davison co-created the Medtronic PEAK PlasmaBlade™, used in 3M+ surgeries and valued at $250M+, and has contributed to more than 25 devices with 14 patents.
Jason Hegener brings 20+ years of engineering leadership across systems like the Stryker PhotonBlade and ConMed EDGE Ablation platform.
Few early-stage teams come with this level of domain-specific success and credibility.
-
This is a team that didn’t step into electrosurgery — they helped define it.
Davison and Hegener have decades of experience designing:
RF-based surgical energy systems
Illumination and visualization technologies
Advanced electrosurgical instruments
Their background directly aligns with CoronaBlade’s core technology, significantly reducing technical and integration risk.ere
-
The founders have repeatedly turned concepts into FDA-cleared, commercially successful devices.
Davison led 16 FDA-cleared product launches at PEAK Surgical, Invuity, and Medtronic.
Hegener has guided five successful 510(k) submissions and helped scale manufacturing and commercialization at major MedTech companies.
This team knows how to reach milestones, navigate regulatory pathways, and build acquirable assets.
-
CoronaBlade benefits from a rare mix of engineering skill, clinical insight, manufacturing knowledge, and strategic execution.
Davison has operated across the MedTech spectrum—from co-founder to VP-level leader—while Hegener has built and scaled complex electrosurgical systems.
Together, they’re equipped to build not just a device, but a long-term surgical platform with enterprise-scale potential. -
CoronaBlade delivers a step-change improvement in open-surgery energy instruments:
Glass-coated blade significantly reduces thermal spread
Cleaner dissection and improved coagulation
More efficient cutting performance
Unlike PlasmaBlade and similar systems requiring $35k–$55k proprietary generators, CoronaBlade works with existing Bovie RF generators already in ORs and ASCs.
-
CoronaBlade follows a clear, lower-risk 510(k) pathway using the FDA-cleared Medtronic PlasmaBlade™ as its predicate device.
This proven predicate enables:
Faster review
Lower regulatory risk
Reduced development cost
Higher probability of clearance
A meaningful strategic advantage for an early-stage MedTech platform.

Market Overview
Open surgery is one of the largest and most stable segments of healthcare, with operating rooms and ambulatory surgery centers (ASCs) performing millions of procedures each year.
As more cases shift to lower-cost outpatient settings, ASCs have quickly grown into one of the most cost-sensitive channels in surgical care.
Nearly all ORs and ASCs already rely on standard Bovie-type RF generators, which power the vast majority of surgical energy instruments used today.
Unlike many other advanced energy devices that require expensive proprietary generators, CoronaBlade is fully compatible with the equipment facilities already own.
For hospitals under budget pressure and ASCs operating on thin margins, eliminating capital cost is one of the most powerful adoption accelerators in the surgical device market.
CoronaBlade’s ability to fit seamlessly into the existing infrastructure dramatically expands its addressable market and positions it for rapid entry across both hospital and ASC settings.

Paul Davison, MS.
CEO & Co-Founder, Lamina Surgical
Paul Davison is a veteran medical-device innovator with more than three decades of experience identifying unmet clinical needs, inventing novel solutions, and scaling breakthrough technologies into global commercial platforms.
As CEO & Co-Founder of Lamina Surgical, he is leading the development of precision surgical instruments that integrate advanced engineering with surgeon-driven design.
Previously, Paul held executive R&D and operating roles at Provisio Medical, Invuity, ConMed, PEAK Surgical, ArthroCare, and Medtronic, where he drove 16 FDA-cleared product launches and developed industry-defining systems including the PEAK PlasmaBlade™, used in millions of procedures worldwide.
An inventor on more than a dozen patents, he brings deep leadership across R&D, IP strategy, regulatory affairs, clinical affairs, and commercialization.
Experienced MedTech leaders with deep expertise in electrosurgical innovation, regulatory strategy, and commercial execution.
Meet the Team

Jason Hegener, BS BIOE
VP Engineering / Electrosurgical Systems
Jason Hegener is an accomplished medical device leader with over 20 years of experience driving the development of innovative surgical technologies from concept to commercial success.
He has a proven track record of guiding five complex 510(k) medical device programs from initial concept through regulatory clearance and commercial launch. Notable product successes include the Stryker PhotonBlade electrosurgical device and the ConMed EDGE Bipolar RF Ablation system.
Jason brings deep expertise in electrosurgical and visualization/imaging technologies, with a specialization in building and leading cross-functional teams across the entire product development lifecycle.